Freyr provides end to end Cosmetic Regulatory Services like formulation & ingredient review, label review, claims review, safety assessment & toxicology services, dossier compilation and market entry support.
Facing issue in account approval? email us at info@ipt.pw
Click to Ckeck Our - FREE SEO TOOLS
Freyr provides End to End Chemical Regulatory Compliance services to health care consumers like product registration, notification, Formulation review and We offer EU REACH and CLP Registration/Dossier Update, Hazard Communication for quick market access.
Freyr iREADY is a technology based Cosmetic Ingredient Database platform and it provides Regulatory compliance support to manufacturers for cosmetics ingredients and management of product formulae in global markets.
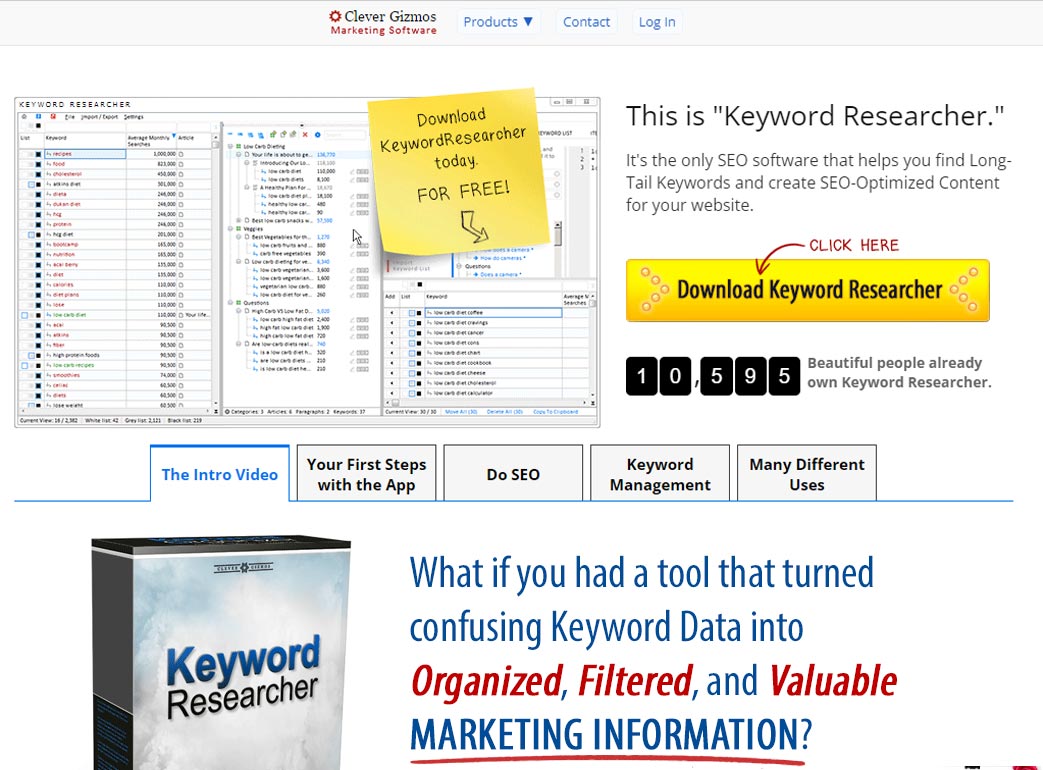
Freyr provides Regulatory Software, Automation and Digital Transformation Solutions to support Global Lifesciences companies.
Freyr SUBMIT Pro is one of the best eCTD software tool which helps Life science companies in various regulatory submissions to global health Authorities.Freyr SUBMIT Pro is one of the best eCTD software tool which helps Life science companies in various regulatory submissions to global health Authorities.
Freyr provides End-to-End Post Brexit regulatory support for Pharma, Medical Devices, Cosmetics and Food Supplements manufacturers who are willing to market their products in United Kingdom (UK) and EU.Freyr provides End-to-End Post Brexit regulatory support for Pharma, Medical Devices, Cosmetics and Food Supplements manufacturers who are willing to market their products in United Kingdom (UK) and EU.
Freyr provides end to end regulatory support for medical device & IVD manufacturers in product registration, notification, classification across the globe.Freyr provides end to end regulatory support for medical device & IVD manufacturers in product registration, notification, classification across the globe.
Freyr provides United Kingdom Responsible Person (UKRP) services to foreign medical device manufacturers in product registration and market entry in the UK and acts as a single point of contact in the Country for liaison with Regulatory Agency.Freyr provides United Kingdom Responsible Person (UKRP) services to foreign medical device manufacturers in product registration and market entry in the UK and acts as a single point of contact in the Country for liaison with Regulatory Agency.
Freyr provides regulatory services and solutions in Thailand to comply with Thailand FDA for pharma, medical device, cosmetics, and food supplement manufacturer companies

Freyr is a Strategic Regulatory partner providing end-to-end Regulatory solutions and services to Innovator pharma companies during their innovator drug development process.
Freyr Artwork 360 is one of the best Artwork Management Software tool which helps Life science companies to simplify the complexity of Artwork Management Process